CEO Ole Thastrup and CBO Maarten van der Linden present 2cureX’s roadmap for commercialization of IndiTreat® at three investor meetings in November: Danish Shareholders’ Association (InvestorUgen), Copenhagen on 12. November, Life Science Day Life Science, Copenhagen on 25. November and Redeye Life Science Day, Stockholm on 26. November.
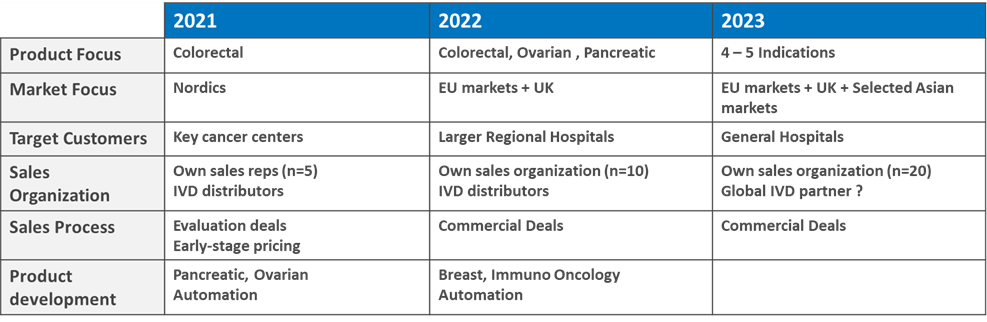
2cureX officially launched its IndiTreat® test for colorectal cancer at the ESMO congress (Eur. Soc. Medical Oncology) in October 2020. As 2cureX is moving forward in its commercialization, the management is now presenting the commercial roadmap for 2021-23 with operational activities.
CEO Ole Thastrup states: “Subsequent to comprehensive pre-launch activities in the first half of 2020 and detailed discussions with oncologists at the ESMO congress, we have finalized a commercialization roadmap on how to implement the IndiTreat® test at European hospitals. The first IndiTreat product is targeting patients with Colorectal Cancer. Later products will cover Ovarian, Pancreatic and Breast cancer patients. Our market analysis has shown that there is a clear need for a functional test like IndiTreat in clinical practice. We now look forward to see IndiTreat® in action at different European cancer clinics.”
To ensure optimal coverage of our target audience 2cureX will expand the commercial team during ’21 and ’22 with several Key Account Managers. Besides strengthening the external salesforce the company will also ensure to bring in capabilities to ensure that the different clinical development projects in Ovarian and Pancreatic Cancer will be brought to commercial readiness and handed over to the sales organization.
Maarten van der Linden, CBO at 2cureX says: “Our ESMO activities and marketing initiatives turned a page in terms of our commercialization efforts and momentum for the company. With a strong product development plan and clinical data around the corner 2cureX is in a very interesting and exciting phase of its journey. I feel very confident that we as a company have grown and are geared ready for a successful outlook ahead.”
We hope that you will have the opportunity to follow our presentation at one of the following events:
Danish Shareholders’ Association (InvestorUgen), Copenhagen on 12. Nov.:
https://www.shareholders.dk/investordagen
Life Science Day Life Science, Copenhagen on 25. Nov.:
https://www.eventbrite.co.uk/e/life-science-investor-konference-den-25112020-tickets-125130700293
Redeye Life Science Day, Stockholm on 26. Nov.:
https://www.redeye.se/events/792760/save-the-date-redeye-life-science-day
For more information about 2cureX, please contact:
Ole Thastrup, CEO
E-post: ot@2curex.com
Telefon: +45 22 11 53 99
www.2curex.com
Certified Adviser
Redeye AB
Telefon: +46 8 121 576 90
E-mail: certifiedadviser@redeye.se
About 2cureX
2cureX has developed the IndiTreat® (Individual Treatment) test. IndiTreat® establishes thousands of 3D micro-tumors that are similar to the patient’s tumor and identifies the treatment that most effectively kills the patient’s tumor. Immediately after the test, the patient can be offered the selected treatment.
IndiTreat® is being clinically validated in clinical studies in colorectal cancer, ovarian cancer, pancreatic cancer and preventive cancer medicine. The clinical programs are conducted at major cancer hospitals in Denmark, Germany, Sweden and United Kingdom.
IndiTreat® is presently being marketed into the European market.
The aspiration is that IndiTreat® becomes a standard tool in Precision Medicine for cancer patients.
The company is listed at the Nasdaq First North Growth Market in Stockholm (symbol: “2CUREX”).